32+ Calculate Specific Heat Capacity
The units of specific heat capacity are Jkg C. Heat capacity is the amount of heat necessary to change the temperature of a substance by 100 C C.

Chapter 5 Thermochemistry Ppt Download
Web Since the solution is aqueous we can proceed as if it were water in terms of its specific heat and mass values.

. Use this calculator to. Web where m is the mass of the substance in grams C is the specific heat capacity and Δ T is the change in temperature during the heat transfer. Web The specific heat of the human body calculated from the measured values of individual tissues is 298 kJ kg1 C1.
Web Heat capacity is the amount of heat required to change the temperature of a given amount of matter by 1C. C is representing the specific heat capacity Q is representing the. Note that both mass and specific.
This is expressed mathematically as. Web Specific heat represents the amount of heat required to change a unit mass of a substance by one degree Celsius. This is 17 lower than the earlier wider used one based.
Web This chemistry video tutorial explains the concept of specific heat capacity and it shows you how to use the formula to solve specific heat capacity problems. Web The units for specific heat can either be joules per gram per degree JgoC Jg o C or calories per gram per degree calgoC calg o C. Web The heat Capacity formula is expressed as the product of mass specific heat and change in the temperature which is mathematically given as.
Web Specific heat is closely related to the concept of heat capacity. Q mcΔT Where Q is the heat. Web If Q amount of heat is supplied or extracted from a substance of mass m to change its temperature from T1 to T2 its specific heat capacity is 1m times the ratio of.
The density of water is approximately 10 gmL so 1000 mL has. The heat capacity of 1 gram of a substance is called its specific heat. 1 they can be used to determine the final temperature attained by a substance when it is either heated or cooled.
Objects with a high specific heat capacity require a greater. Web Heat capacity is a property that describes how much energy is needed to change the temperature of a material. Web The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled.
Web Specific Heat Capacity C or S - The quantity of heat required to raise the temperature of a substance by one degree Celsius is called the specific heat capacity of the. Web Specific Heat Formula. Web Specific Heat Capacity c m This is the amount of heat required to raise a substance or material by one unit of mass and one unit of temperature.
Web Since most specific heats are known Table 3121 312. C frac QmtimesDelta T Whereas. Q m c ΔT where.
Web It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree Celsius. The heat capacity formula is.
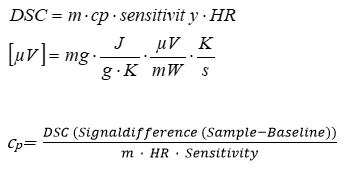
Specific Heat Capacity Cp Netzsch Analyzing Testing

Calculate The Specific Heat Capacity At Constant Volume For A Gas Given Specific Heat Capacity At Youtube

Gcse Physics Revision Specific Heat Capacity Youtube
13 2 Specific Heat Physics Libretexts
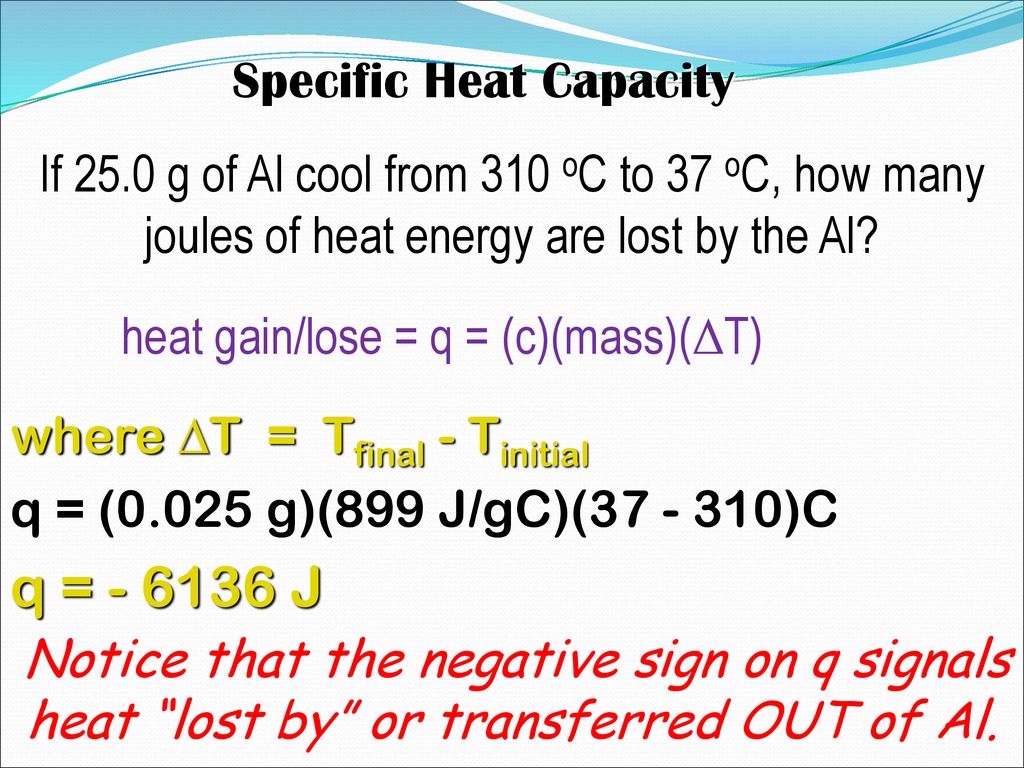
Or The Amount Of Energy Needed To Heat Substances Up Ppt Download
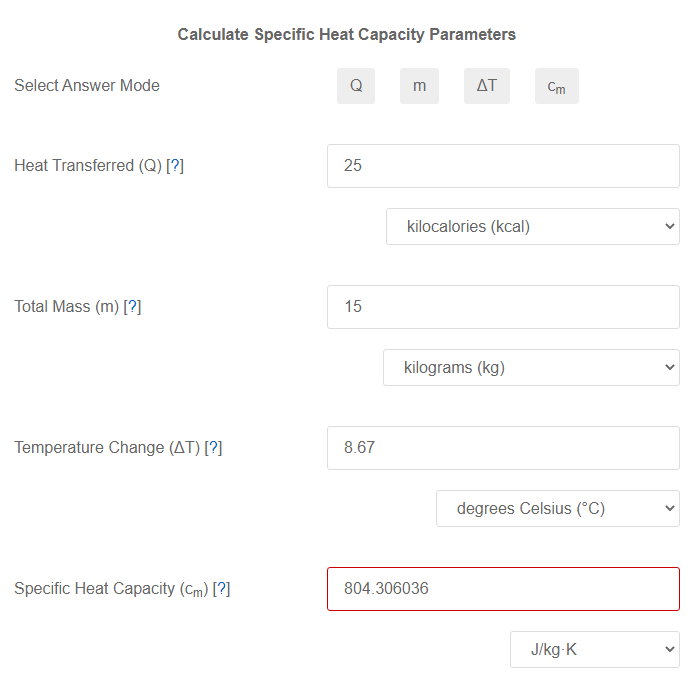
Specific Heat Capacity Calculator
What Is The Specific Heat Capacity Of 50 Calorie Heat To 30g Of A Substance Where Its Temperature Increases From 15 Degrees Celsius To 40 Degrees Celsius Quora

Solved How Much Heat In Joules Is Required To Raise The Temperature Of 1 82 Kg Of Water By 3 32 Degrees C Specific Heat Of Water Is 4185 J Kg C
When Equal Masses Of Copper And Lead Are Heated At The Same Temperature The Mass Of Copper Is At A Lower Temperature Which Has A Higher Specific Heat Capacity Quora

Chapter 17 Thermochemistry Ppt Download
Specific Heat Online Unit Converter

Specific Heat Capacity Overview Calculator
How Much Heat Energy Is Required To Melt 5 Kg Of Ice Specific Latent Heat Of Ice Is 336 J G Quora
30 Gm Of Ice At 14 C Is Added To 200 Gm Of Water At 25 C What Is The Equilibrium Temperature Specific Heat Of Ice 0 5cal G C Specific Heat Of Water 1

Solved Use The Data In The Table To Answer The Following Questions Use 1 Answer Transtutors

Specific Heat Capacity
What Is The Energy Required To Make 500 Gram Water To Change Its Temperature Form 20c To 80c If The Specific Heat Capacity Of Water Is 4200 J Kg C Quora